Combined remediation of As(Ⅲ)-contaminated soils by pre-oxidation, stabilization and solidification
-
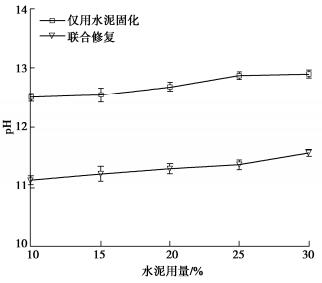
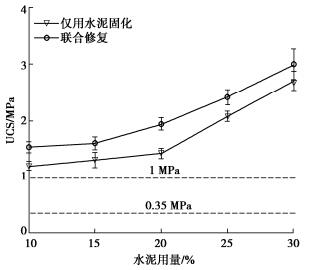
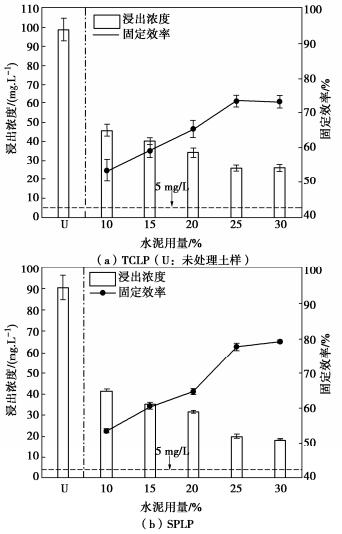
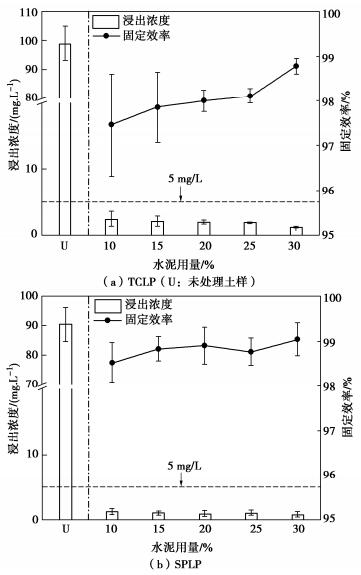
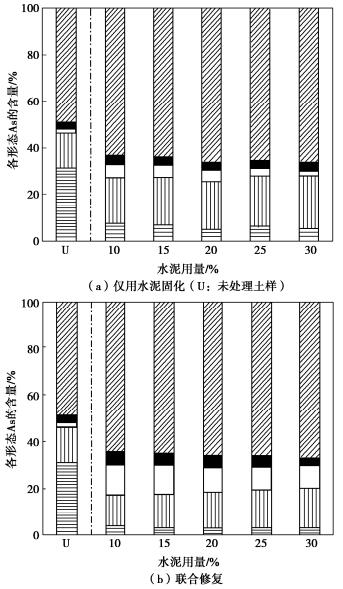
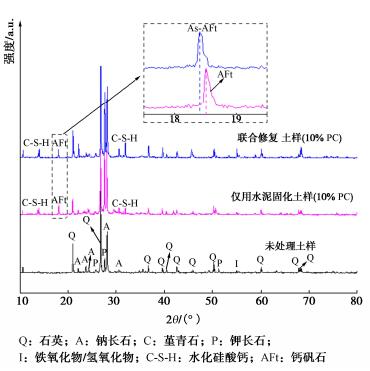
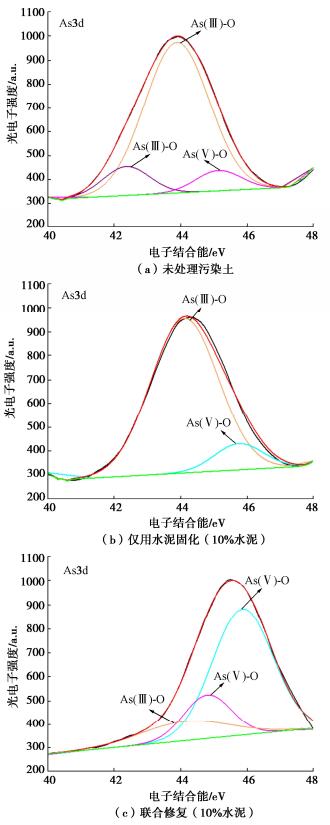
摘要: 固化/稳定化是重金属污染土治理的最常用方法,但因为As(Ⅲ)的迁移性强,固化处理As(Ⅲ)污染土的效果欠佳。提出了As(Ⅲ)污染土的预氧化—稳定化—固化联合修复方法,首先利用Fenton试剂将土中As(Ⅲ)氧化为迁移性弱的As(Ⅴ),再用FeCl3稳定As(Ⅴ),最后通过水泥固化进一步固定As(Ⅴ)。通过无侧限抗压强度试验、毒性浸出试验(TCLP)、合成沉降浸出试验(SPLP)、pH测试、连续萃取试验和光谱分析研究了联合修复的效果和机理。结果表明,Fenton预氧化能够有效提高修复效率,当Fe与As的摩尔比为1︰1、添加10%水泥时,TCLP和SPLP得到As的浸出毒性分别低至2.51,1.33 mg/L,修复效率分别高达97.46%,98.53%;FeCl3能促进水泥的水化并改善固化体的孔隙结构,提高固化体的强度;修复方法可将As转化为更稳定的形态,有效降低As的潜在环境风险;大多数As可以与铁的水合氧化物结合而被固定,但随着水泥用量增加pH增加,Fe-As的结合减弱,导致部分As释放;光谱分析结果表明,联合修复可以将土中92.5%的As(Ⅲ)转化为As(Ⅴ),并通过水化硅酸钙的固封和钙矾石的离子交换将As固定。该研究为As(Ⅲ)污染土的高效修复提供了新的视角。Abstract: The solidification/stabilization (S/S) is the most popular method for treatment of heavy metal-contaminated soils, however, the S/S treatment of As(Ⅲ)-contaminated soils is not effective due to the high mobility of As(Ⅲ). A combined remediation technique is proposed, in which As(Ⅲ) is first oxidized to As(Ⅴ) by the Fenton reagent, then stabilized by FeCl3 and finally stabilized by cement. The unconfined compressive strength tests, toxicity characteristic leaching procedure (TCLP), synthetic precipitation leaching procedure (SPLP), pH measurements, sequential extraction procedure and spectroscopic investigations are carried out to investigate the effects and mechanism of the proposed technique. The results show that the Fenton pre-oxidation process significantly improves the remediation efficiency. Under an Fe-to-As molar ration of 1:1 and a cement dosage of 10%, the leaching toxicity of As in TCLP and SPLP is as low as 2.51 and 1.33 mg/L, and the immobilization efficiency reaches 97.46% and 98.53%, respectively. The hydration degree of the cement and the pore structure of the curing body are improved by FeCl3 and therefore the strength increases. The combined remediation can transform As to more stable phases and effectively reduce the potential environmental risk. The majority of As is bound to hydrous oxides of Fe, but an increase in pH due to the increasing cement dosage will affect the Fe-As binding and cause potential release of As. The spectroscopic investigations show that the proposed remediation can transform 92.5% of As(Ⅲ)to As(Ⅴ) and immobilize As by the encapsulation of calcium silicate hydrate and the ion exchange of ettringite. This study provides a new insight into the effective remediation of As(Ⅲ)-contaminated soils.
-
Keywords:
- As(Ⅲ)-contaminated soil /
- pre-oxidation /
- stabilization /
- solidification /
- leaching toxicity
-
0. 引言
瑞利波传播过程中主要引起水平方向振动,这种振动与建筑物的自然频率相近时,会引起共振,导致更大的破坏。为进一步明确瑞利波传播特性和衰减规律,大量学者对其进行理论分析[1]、数值模拟[2]和试验研究[3]。瑞利波在介质中的传播受多种因素影响,主要包括介质的弹性参数、密度、重力以及分层结构等[4]。
桩基在瑞利波作用下的动力响应的研究成为抗震过程中的重要组成部分。Yang等[5]在考虑桩顶柔性约束的情况下,研究了非饱和土-桩体系在瑞利波作用下的动力响应。此外Cai等[6]采用刚性排桩作为隔离瑞利波的方法,考虑了软土地质所产生的影响。
通过以上讨论发现:考虑竖向荷载影响的瑞利波作用下桩水平动力响应分析比较复杂。且对于软土地基中桩在瑞利波作用下的动力响应的研究也相对较少。因此,本文建立考虑竖向荷载对瑞利波作用下饱和软土地基中桩的水平动力响应影响的计算模型。研究结果为瑞利波作用下桩基动力分析和设计提供理论指导。
1. 计算模型
1.1 问题描述
在瑞利波的作用下饱和土-桩体系的数学模型如图 1所示,其中桩长为L,半径为ra,截面积为Ap,密度为ρp,杨氏模量为Ep,剪切模量为Gp。桩体模型简化为桩端为固定边界条件的Timoshenko梁,桩顶视为质量为M0的刚性块体。桩周饱和土视为线弹性材料,泊松比、阻尼比和剪切模量分别取为ν0,ε0,s和Gs。其中瑞利波的作用方式是以波的形式穿过土体传播到桩体上。
1.2 基本方程
根据Biot’s理论,运动方程可以表示为
σij,j=ρs¨ui+ρf¨wi, (1) −pf,i=ρf¨ui+m1¨wi+r1˙wi。 (2) 式中:ui为土体位移;wi为流体位移;λ,μ为拉梅常数;m1=ρf/n为孔隙中流体密度与孔隙率的比值;r1=ρfg/kd,kd为土壤达西渗透系数,pf为孔隙中流体压力;α和M分别为两相材料的Biot’s参数。
基于Timoshenko理论,建立桩段控制微分方程[7]:
ApGpk0(∂2up∂z2−∂θp∂z)+ρpAp∂2up∂t2+(kh+ich)(up−urcosθ)=0, (3) ApGpk0(∂up∂z−θp)−ρpIp∂2up∂t2−EpIp∂2θp∂z2+ApP∂up∂z=0。 (4) 式中:up(z,t)=ˉup(z)eiωt,θp(z,t)=ˉθp(z)eiωt分别为桩的水平位移和转角;k0为Timoshenko梁理论中的修正剪切因子;P为由上部结构质量引起的竖向荷载。
2. 方程的求解
由式(2)得:
wr=ω2ρfurϑ−1ϑ∂pf∂r, wθ=ω2ρfuθϑ−1ϑ∂pfr∂θ。 (5) 式中:η2a=2/(1−ν),ϑ=iωρfg/kd−m1ω2。
将式(5)代入式(1),(2)结合本构方程得
η2b∂∂r[1r(∂(rur)∂r+∂uθ∂θ)]−1r2∂∂θ[∂(ruθ)∂r−∂ur∂θ]+∂2ur∂z2+c1ρsω2Gsur−c2∂pf∂r=0, (6) ∂∂r[1r(∂(ruθ)∂r−∂ur∂θ)]+η2b1r2∂∂θ[∂(rur)∂r+∂uθ∂θ]+∂2uθ∂z2+c1ρsω2Gsuθ−c2∂pfr∂θ=0。 (7) 式中:c1=1+ω2ρ2fρsϑ;c2=α2μ1−2ν1−ν+ω2ρfμsϑ;η2b=2−ν1−ν
忽略流体的垂直位移:
(ω2ρfϑ−α1−2ν01−ν0)(∂ur∂r+urr+1r∂uθ∂θ)=1ϑ∇2pf+(α2λν01−ν0+1M)pf。 (8) 水平位移和切向位移用两个势函数Φ和Ψ表示为
ur=∂ϕ∂r+1r∂ψ∂θ, uθ=1r∂ϕ∂θ−∂ψ∂r。 (9) 将式(9)代入式(6)~(8),联立得
η2b∇2ϕ+∂2ϕ∂z2+c1ρsGsω2ϕ=c2pf, (10) ∇2ψ+∂2ψ∂z2+c1ρsGsω2ψ=0, (11) ∇2pf+(ϑα2λν01−ν0+ϑM)pf=(ω2ρf−αϑ1−2ν01−ν0)∇2ϕ。 (12) 采用分离变量法,设ϕ(r,θ,z)=ϕ1(r,θ)Z(z),假设φ(r,θ,z)=φ1(r,θ)Z(z),d2Zdz2+a2Z=0,将Z(z)表示为
Z(z)=B0cos(az)+B1sin(az)。 (13) 联立式(10)~(12)并将式(13)代入得
∇2ψ1−[a2−c1ρsGsω2]ψ1=0。 (14) (∇2−ζ211)(∇2−ζ212)ϕ1=0 (15) 这里设:d11=c1η2bρsGsω2+ϑM−a2η2b+c2αϑη2b1−2ν01−ν0−c2ω2ρfη2b+ϑα2λν01−ν0,d12=ϑη2b[c1ρsGsω2(α2λν01−ν0+1M)−a2α2λν01−ν0−a2M], ζ211=−d11+√d211−4d122, ζ212=−d11−√d211−4d122,vs=√Gsρs,从而得到
(∇2+k2a1)(∇2+k2a2)ϕ1=0, (16) (∇2+k2b1)ψ1=0。 (17) 通过算子分解理论和分离变量法得:
ϕ1=As1exp(−s1rsinθ−ikRrcosθ)+As2exp(−s2rsinθ−ikRrcosθ), (18) ψ1=Bsexp(−γrsinθ−ikRrcosθ)。 (19) 式中:As1,As2,Bs分别为与边界条件有关的待定常数;kR,VR=ω/kR分别为瑞利波的复波速和相速度;si=√k2R−k2ai(i=1, 2)和γ=√k2R−k2b1,分别为压缩波和剪切波对应的衰减指数;k2a1=−ζ211,k2a2=−ζ212,k2b1= −a2+κ1ρsGsω2。
因此由式(13),结合边界条件即τrz|z=0=0,ur|z=L=uθ|z=L=0可知
B0=B1=0且cos(aL)=0 , am=(2m−1)π2L,m=1,2,3 。} (20) φ=[As1exp(−s1rsinθ−ikRrcosθ)+As2exp(−s2rsinθ−ikRrcosθ)]sin(amz), (21) ψ=Bsexp(−γrsinθ−ikRrcosθ)sin(amz)。 (22) 将式(21)代入式(10)得
pf=[−a2mc2+c1c2(ωVs)2][As1exp(−s1rsinθ−ikRrcosθ)+As2exp(−s2rsinθ−ikRrcosθ)]sin(amz)+η2bc2(s21−k2R)As1exp(−s1rsinθ−ikRrcosθ)sin(amz)+η2bc2(s22−k2R)As2exp(−s2rsinθ−ikRrcosθ)sin(amz)。 (23) 通过式(9)结合本构方程及式(21),(22)并代入边界条件τrz|z=0=0,uθ|z=L=0可知
As2=d1As1,Bs=d2As1。 (24) 联立式(9)即可求得位移转角ur、uθ表达式。
因此自由场饱和土在瑞利波作用下位移由体积平均原理得
ˉur=(1−n)ur+nwr, (25) ˉuθ=(1−n)uθ+nwθ。 (26) 3. 瑞利波作用下桩的动力响应
3.1 水平阻力
桩在瑞利波作用下的动力响应由桩周土体决定,采用动力Winkler模型来描述饱和土桩体系的水平动力响应,进而计算单位桩长上的水平阻力[8-9]。因此设单位桩长上的水平阻力为
qh=(kh+ich)uˉp。 (27) 式中:kh和ch分别为Winkler模型中弹簧刚度和阻尼系数。
3.2 考虑竖向荷载影响的桩的动力响应
将式(25),(27)代入式(3),(4)并省略因子eiωt得
d4ˉupdz4+Wd2ˉupdz2+Jˉup=H1As1sin(amz), (28) d4ˉθpdz4+Wd2ˉθpdz2+Jˉθp=H2As1cos(amz)。 (29) 其中,W=ρPω2Ep+ρPω2k0Gp+ApPEpIp−(kh+ich)k0ApGp,J= ρ2pω4k0GpEp−ρPApω2EpIp−(ρPω2k0ApGpEp−1EpIp)(kh+ich)
方程(28)、(29)对应的解设为
ˉup=M1cos(λ1z)+M2sin(λ1z)+M3ch(λ2z)+M4sh(λ2z)+b1As1sin(amz), (30) ˉθp=M1χ1sin(λ1z)+M2χ2cos(λ1z)+M3χ3sh(λ2z)+M4χ4ch(λ2z)+b2As1cos(amz), (31) 式中:λ1=√W+√W2−4J2,λ2=√−W+√W2−4J2, Mi(1,2,3,4)为与桩的边界条件有关的待定系数。其中χ1=−χ2=−k0ApGpλ1+ApPλ1k0ApGp+ρpIpω2+EpIpλ21, χ3= χ4=k0ApGpλ2+ApPλ2k0ApGp+ρpIpω2−EpIpλ21,b1=H1a4m−Wa2m+J,b2=H2a4m−Wa2m+J。
沿桩身的弯矩和剪力可由弹性力学推导为
ˉMp=EPIP[M1χ1λ1cos(λ1z)−M2χ2λ1sin(λ1z)+M3χ3λ2ch(λ2z)+M4χ4λ2sh(λ2z)−b2amAs1sin(amz)]。 (32) ˉQp=k0APGP[−M1(λ1+χ1)sin(λ1z)+M2(λ1−χ2)⋅cos(λ1z)+M3(λ2−χ3)sh(λ2z)+M4(λ2−χ4)ch(λ2z)+(b1am−b2)As1cos(amz)]。 (33) 桩顶柔性状态下,桩端处于固定状态,桩的边界条件为:
¯Mp(z)=K1¯θc+K2[¯θp(0)−¯θc], z=0 , ¯Qp(z)−ApP¯θp(z)+ω2M0¯up(z)=0,z=0 , ¯up(z)=0, ¯θp(z)=0,z=L 。} (34) 将边界条件代入桩体水平位移、旋转角度沿桩身的弯矩和剪力表达式,由此可以导出所有未确定的待定参数Mi(i=1, 2, 3, 4),As1。
4. 数值分析及讨论
通过数值算例验证,分析竖向荷载及桩顶柔性约束下各参数对于桩身位移、转角和弯矩的影响。各相关参数属性:ρs=2.7×103 kg/m3,ρf=2.2×103 kg/m3,Ea=2×109 Pa,ν0=0.4,n=0.4,α=0.9,kd=1×10-8 m/s,Ks=3.6×1010 Pa,Kf=2×109 Pa,Gs=2.5×106 Pa,d=1 m,ra=0.5 m,L=20 m,ρp=2.5×103 kg/m3,Ep= 2.5×1010 Pa,k0=0.75,Ip=π/64,νp=0.2,Ap=0.25π,M0=1×105 kg,P=10×106 Pa。引入无量纲频率a0=ωLa/vs。
4.1 验证
为验证模型的准确性,将Makris[10]解与模型进行比较。在相同条件下,对本文的计算模型进行验证。图 2将水平位移与Makris[10]解的结果进行比较。可以看出两种解法有较高的一致性。
4.2 参数分析
图 3,4给出了瑞利波作用下,竖向荷载对单桩的水平动力响应的影响,其研究了桩长、竖向荷载大小和无量纲频率对桩基水平振动的影响。从图 3可以看出,随着竖向荷载的增加,位移、转角和弯矩都在增大,且增加的趋势也越来越大。随着深度的增加,竖向荷载改变所引起的变化不再明显,最终趋于稳定。
图 4显示了垂直载荷对位移、转角和弯矩的影响。当频率等于固有频率时,发生共振。在共振区,当竖向荷载较小时,随竖向荷载的增大,桩顶位移和桩端弯矩整体呈增大趋势。随无量纲频率的增大,竖向荷载的改变引起水平位移和转角的变化将不再明显。
5. 结论
考虑竖向荷载对瑞利波作用下饱和软土中单桩结构水平动力响应的影响,建立瑞利波作用下饱和软土中桩顶柔性约束下单桩水平动力响应的计算模型。通过数值计算结果,得出以下3点结论。
(1)随深度的增加,位移和转角先减小后增大;弯矩整体呈减小趋势;最终接近桩端固结端时桩的水平动力响应趋于稳定。
(2)随无量纲频率的增加,位移、转角和弯矩在共振区发生共振后最终趋于稳定。
(3)随竖向荷载的增加,位移、转角和弯矩都在增大。但是这种改变所引起的变化随着深度的增加不再明显,最终趋于稳定。
-
表 1 试验用土的金属含量
Table 1 Metal contents in test soil
(单位:mg/kg) 金属元素 As Mn Al Fe Ca Mg 含量 8.9 679 67700 13100 9650 2470 表 2 连续萃取试验步骤
Table 2 Sequential extraction procedure
形态 萃取剂 萃取条件 固液比 洗涤步骤 F1 (NH4)2SO4 (0.05 mol/L) 震荡4 h,20 ℃ 1︰25 F2 (NH4)H2PO4 (0.05 mol/L) 震荡16 h,20 ℃ 1︰25 F3 草酸铵缓冲液(0.2 mol/L);pH 3.25 暗处震荡4 h,20 ℃ 1︰25 草酸铵溶液(0.2 mol/L);pH 3.25;固液比1︰12.5;暗处震荡10 min F4 草酸铵缓冲液(0.2 mol/L);+抗坏血酸(0.1 mol/L) pH 3.25 开盖和非避光条件下,96 ℃水浴处理30 min 1︰25 草酸铵溶液(0.2 mol/L);pH 3.25;固液比1︰12.5;暗处震荡10 min F5 HNO3/H2O2 微波消解 1︰50 -
[1] LOUKOLA-RUSKEENIEMI K, MÜLLER I, REICHEL S, et al. Risk management for arsenic in agricultural soil-water systems: lessons learned from case studies in Europe[J]. Journal of Hazardous Materials, 2022, 424: 127677. doi: 10.1016/j.jhazmat.2021.127677
[2] MOHAMMED ABDUL K S, JAYASINGHE S S, CHANDANA E P S, et al. Arsenic and human health effects: a review[J]. Environmental Toxicology and Pharmacology, 2015, 40(3): 828-846. doi: 10.1016/j.etap.2015.09.016
[3] SONG P P, YANG Z H, ZENG G M, et al. Electrocoagulation treatment of arsenic in wastewaters: a comprehensive review[J]. Chemical Engineering Journal, 2017, 317: 707-725. doi: 10.1016/j.cej.2017.02.086
[4] FERREIRA R T, SILVA A R C, PIMENTEL C, et al. Arsenic stress elicits cytosolic Ca2+ bursts and Crz1 activation in Saccharomyces cerevisiae[J]. Microbiology, 2012, 158(9): 2293-2302. doi: 10.1099/mic.0.059170-0
[5] TSANG D C W, HARTLEY N R. Metal distribution and spectroscopic analysis after soil washing with chelating agents and humic substances[J]. Environmental Science and Pollution Research, 2014, 21(5): 3987-3995. doi: 10.1007/s11356-013-2300-y
[6] 查甫生, 刘晶晶, 许龙, 等. 水泥固化重金属污染土干湿循环特性试验研究[J]. 岩土工程学报, 2013, 35(7): 1246-1252. http://manu31.magtech.com.cn/Jwk_ytgcxb/CN/abstract/abstract15114.shtml ZHA Fusheng, LIU Jingjing, XU Long, et al. Cyclic wetting and drying tests on heavy metal contaminated soils solidified/stabilized by cement[J]. Chinese Journal of Geotechnical Engineering, 2013, 35(7): 1246-1252. (in Chinese) http://manu31.magtech.com.cn/Jwk_ytgcxb/CN/abstract/abstract15114.shtml
[7] 杜延军, 金飞, 刘松玉, 等. 重金属工业污染场地固化/稳定处理研究进展[J]. 岩土力学, 2011, 32(1): 116-124. doi: 10.3969/j.issn.1000-7598.2011.01.019 DU Yanjun, JIN Fei, LIU Songyu, et al. Review of stabilization/solidification technique for remediation of heavy metals contaminated lands[J]. Rock and Soil Mechanics, 2011, 32(1): 116-124. (in Chinese) doi: 10.3969/j.issn.1000-7598.2011.01.019
[8] PARK J Y, KANG W H, HWANG I. Hexavalent chromium uptake and release in cement pastes[J]. Environmental Engineering Science, 2006, 23(1): 133-140. doi: 10.1089/ees.2006.23.133
[9] LI J S, BEIYUAN J, TSANG D C W, et al. Arsenic-containing soil from geogenic source in Hong Kong: Leaching characteristics and stabilization/solidification[J]. Chemosphere, 2017, 182: 31–39. doi: 10.1016/j.chemosphere.2017.05.019
[10] PAN S Y, SHAH K J, CHEN Y H, et al. Deployment of accelerated carbonation using alkaline solid wastes for carbon mineralization and utilization toward a circular economy[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(8): 6429-6437.
[11] 张亭亭, 李江山, 王平, 等. FeSO4对铬污染土的稳定特性及风险评价试验研究[J]. 岩土力学, 2019, 40(10): 3928-3936. https://www.cnki.com.cn/Article/CJFDTOTAL-YTLX201910027.htm ZHANG Tingting, LI Jiangshan, WANG Ping, et al. Stabilization characteristics and risk assessment of hexavalent chromium-contaminated soils by ferrous sulfate treatment[J]. Rock and Soil Mechanics, 2019, 40(10): 3928-3936. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-YTLX201910027.htm
[12] LI L, WANG X, ZHOU Y Y, et al. Effectiveness and limitation of A-nZVI for restoration of a highly As-contaminated soil[J]. Journal of Cleaner Production, 2021, 284: 124691. doi: 10.1016/j.jclepro.2020.124691
[13] WANG Y N, TSANG Y F, WANG H W, et al. Effective stabilization of arsenic in contaminated soils with biogenic Manganese oxide (BMO) materials[J]. Environmental Pollution, 2020, 258: 113481. doi: 10.1016/j.envpol.2019.113481
[14] ZHANG M Y, WANG Y, ZHAO D Y, et al. Immobilization of arsenic in soils by stabilized nanoscale zero-valent iron, iron sulfide (FeS), and magnetite (Fe3O4) particles[J]. Chinese Science Bulletin, 2010, 55(4): 365-372. http://library.rcees.ac.cn/bitstream/311016/21185/1/Immobilization%20of%20arsenic%20in%20soils%20by%20stabilized%20nanoscale%20zero-valent%20iron%2c%20iron%20sulfide%20(FeS)%2c%20and%20magnetite%20(Fe3%20O4%20)%20particles%20.pdf
[15] MOON D H, WAZNE M, YOON I H, et al. Assessment of cement kiln dust (CKD) for stabilization/solidification (S/S) of arsenic contaminated soils[J]. Journal of Hazardous Materials, 2008, 159(2/3): 512-518. http://www.onacademic.com/detail/journal_1000034083474110_1577.html
[16] XENIDIS A, STOURAITI C, PAPASSIOPI N. Stabilization of Pb and As in soils by applying combined treatment with phosphates and ferrous iron[J]. Journal of Hazardous Materials, 2010, 177(1/2/3): 929-937. http://www.researchgate.net/profile/Christina_Stouraiti/publication/41190010_Stabilization_of_Pb_and_As_in_soils_by_applying_combined_treatment_with_phosphates_and_ferrous_iron/links/004635165087c9a5f1000000
[17] 赵慧敏. 铁盐-生石灰对砷污染土壤固定/稳定化处理技术研究[D]. 北京: 中国地质大学(北京), 2010. ZHAO Huimin. Study on Solidification/Stabilization Technology of Arsenic Contaminated Soils Using Molysite and Quicklime[D]. Beijing: China University of Geosciences, 2010. (in Chinese)
[18] NAZARI A M, RADZINSKI R, GHAHREMAN A. Review of arsenic metallurgy: treatment of arsenical minerals and the immobilization of arsenic[J]. Hydrometallurgy, 2017, 174: 258-281. doi: 10.1016/j.hydromet.2016.10.011
[19] HUANG Y F, GAO M L, DENG Y X, et al. Efficient oxidation and adsorption of As(Ⅲ) and As(Ⅴ) in water using a Fenton-like reagent, (ferrihydrite)-loaded biochar[J]. The Science of the Total Environment, 2020, 715: 136957. doi: 10.1016/j.scitotenv.2020.136957
[20] BURBANO A A, DIONYSIOU D D, SUIDAN M T, et al. Oxidation kinetics and effect of pH on the degradation of MTBE with Fenton reagent[J]. Water Research, 2005, 39(1): 107-118. doi: 10.1016/j.watres.2004.09.008
[21] ZHANG W J, LIN M F. Influence of redox potential on leaching behavior of a solidified chromium contaminated soil[J]. Science of the Total Environment, 2020, 733: 139410. doi: 10.1016/j.scitotenv.2020.139410
[22] WENZEL W W, KIRCHBAUMER N, PROHASKA T, et al. Arsenic fractionation in soils using an improved sequential extraction procedure[J]. Analytica Chimica Acta, 2001, 436(2): 309-323. doi: 10.1016/S0003-2670(01)00924-2
[23] 陈蕾, 杜延军, 刘松玉, 等. 水泥固化铅污染土的基本应力-应变特性研究[J]. 岩土力学, 2011, 32(3): 715-721. doi: 10.3969/j.issn.1000-7598.2011.03.013 CHEN Lei, DU Yanjun, LIU Songyu, et al. Experimental study of stress-strain properties of cement treated lead-contaminated soils[J]. Rock and Soil Mechanics, 2011, 32(3): 715-721. (in Chinese) doi: 10.3969/j.issn.1000-7598.2011.03.013
[24] AL-KINDI G. Evaluation the solidification/stabilization of heavy metals by Portland cement[J]. Journal of Ecological Engineering, 2019, 20(3): 91-100. doi: 10.12911/22998993/99739
[25] LI J S, WANG L, CUI J L, et al. Effects of low-alkalinity binders on stabilization/solidification of geogenic As-containing soils: Spectroscopic investigation and leaching tests[J]. Science of the Total Environment, 2018, 631/632: 1486-1494. doi: 10.1016/j.scitotenv.2018.02.247
[26] YANG K, KIM B C, NAM K, et al. The effect of arsenic chemical form and mixing regime on arsenic mass transfer from soil to magnetite[J]. Environmental Science and Pollution Research, 2017, 24(9): 8479-8488. doi: 10.1007/s11356-017-8510-y
[27] ZHA F S, LIU C M, KANG B, et al. Acid rain leaching behavior of Zn-contaminated soils solidified/stabilized using cement–soda residue[J]. Chemosphere, 2021, 281: 130916. doi: 10.1016/j.chemosphere.2021.130916
[28] 查甫生, 许龙, 崔可锐. 水泥固化重金属污染土的强度特性试验研究[J]. 岩土力学, 2012, 33(3): 652-656, 664. doi: 10.3969/j.issn.1000-7598.2012.03.002 ZHA Fusheng, XU Long, CUI Kerui. Strength characteristics of heavy metal contaminated soils stabilized/solidified by cement[J]. Rock and Soil Mechanics, 2012, 33(3): 652-656, 664. (in Chinese) doi: 10.3969/j.issn.1000-7598.2012.03.002
[29] KARAK T, ABOLLINO O, BHATTACHARYYA P, et al. Fractionation and speciation of arsenic in three tea gardens soil profiles and distribution of As in different parts of tea plant (Camellia sinensis L. )[J]. Chemosphere, 2011, 85(6): 948-960. doi: 10.1016/j.chemosphere.2011.06.061
[30] CAO Y Z, GUO L P, CHEN B, et al. Modeling early age hydration kinetics and the hydrated phase of cement paste blended with chloride and sulfate[J]. Construction and Building Materials, 2020, 261: 120537. doi: 10.1016/j.conbuildmat.2020.120537
[31] COUSSY S, PAKTUNC D, ROSE J, et al. Arsenic speciation in cemented paste backfills and synthetic calcium-silicate-hydrates[J]. Minerals Engineering, 2012, 39: 51-61. doi: 10.1016/j.mineng.2012.05.016
[32] CHRYSOCHOOU M, DERMATAS D. Evaluation of ettringite and hydrocalumite formation for heavy metal immobilization: literature review and experimental study[J]. Journal of Hazardous Materials, 2006, 136(1): 20-33. doi: 10.1016/j.jhazmat.2005.11.008
[33] ZHANG M T, YANG C H, ZHAO M, et al. Immobilization potential of Cr(Ⅵ) in sodium hydroxide activated slag pastes[J]. Journal of Hazardous Materials, 2017, 321: 281-289. doi: 10.1016/j.jhazmat.2016.09.019
[34] KIM E J, LEE J C, BAEK K. Abiotic reductive extraction of arsenic from contaminated soils enhanced by complexation: arsenic extraction by reducing agents and combination of reducing and chelating agents[J]. Journal of Hazardous Materials, 2015, 283: 454-461. doi: 10.1016/j.jhazmat.2014.09.055
[35] ZHANG S J, LI X Y, CHEN J P. An XPS study for mechanisms of arsenate adsorption onto a magnetite-doped activated carbon fiber[J]. Journal of Colloid and Interface Science, 2010, 343(1): 232-238. doi: 10.1016/j.jcis.2009.11.001
[36] SUN M, ZHANG G, QIN Y H, et al. Redox conversion of chromium(Ⅵ) and arsenic(Ⅲ) with the intermediates of chromium(Ⅴ) and arsenic(Ⅳ) via AuPd/CNTs electrocatalysis in acid aqueous solution[J]. Environmental Science & Technology, 2015, 49(15): 9289-9297. doi: 10.1021/acs.est.5b01759
[37] CHEN Z H, JIN J Y, SONG X J, et al. Redox conversion of arsenite and nitrate in the UV/quinone systems[J]. Environmental Science & Technology, 2018, 52(17): 10011-10018. http://www.ncbi.nlm.nih.gov/pubmed/30063337
[38] KOPPENOL W H, STANBURY D M, BOUNDS P L. Electrode potentials of partially reduced oxygen species, from dioxygen to water[J]. Free Radical Biology and Medicine, 2010, 49(3): 317-322. doi: 10.1016/j.freeradbiomed.2010.04.011
[39] HERNÁNDEZ-FLORES H, PARIONA N, HERRERA-TREJO M, et al. Concrete/maghemite nanocomposites as novel adsorbents for arsenic removal[J]. Journal of Molecular Structure, 2018, 1171: 9-16. doi: 10.1016/j.molstruc.2018.05.078




 下载:
下载: