Research advances in chemical interaction mechanism between highly compacted bentonite and pore solution
-
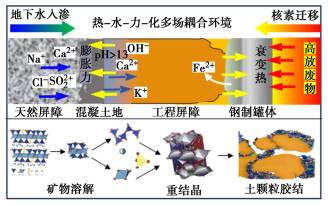
摘要: 高压实膨润土作为高放废物深地质处置首选缓冲/回填材料,在处置库近场热-水-力-化多场耦合环境中必然会与孔隙溶液发生化学作用,使蒙脱石溶解甚至相变,导致工程屏障缓冲性能衰减失效。在全面阐述孔隙溶液化学作用对高压实膨润土缓冲性能影响规律的基础上,系统总结了高压实膨润土与孔隙溶液化学作用机制的最新研究成果。分析表明,层状蒙脱石溶解相变为架状矿物是导致膨润土比表面积、相对质量密度、持水性能、膨胀性能、防渗性能等发生衰减的关键因素。孔隙溶液对高压实膨润土的化学作用机制包括矿物化学相变和化学胶结作用。其中,矿物化学相变与孔隙溶液化学组成、pH、温度和活性催化离子有关,可分为同晶相变和溶解重结晶两种机制;化学胶结与膨润土干湿循环产生盐渍沉淀填充和硅铝酸盐胶凝物胶结作用密切相关。膨润土中矿物的溶解速率不仅与自身反应表面积、所受应力和溶解平衡有关,还与孔隙溶液的化学组成、pH、温度和活性催化离子等环境因素密切相关。针对膨润土内的反应体系,进一步明确化学反应参数、胶结作用影响和多场耦合反应模型仍是今后膨润土化学演化需要深入研究的重点方向。Abstract: The highly compacted bentonite, as the preferred buffer/backfill materials, is inevitably subjected to chemical erosion in the T-H-M-C environment of the high-level radioactive waste repositories, leading to dissolution or phase transition of smectite, and diminishing the buffer performance. The latest researches on the chemical mechanism are summarized on the basis of reviewing the effects of the solution on the buffer performance of the compacted bentonite. The analysis indicates that the dissolution or phase transformation of layered smectite into a framework mineral is the key factor leading to the attenuation of the specific surface area, density, water retention, swelling and permeation resistance of bentonite. The chemical interaction mechanisms include mineral phase transformation and chemical cementation. The phase transformation of minerals is influenced by chemical composition, pH, temperature and catalytic ions of the pore solution, and can be divided into isomorphous phase transformation and recrystallization. The chemical cementation associates with saline precipitate filling and the cementation of aluminosilicate gelation during wetting-drying cycles. The dissolution rate of minerals in bentonite is influenced by both the intrinsic factors like surface area and stress, and the extrinsic factors including pore solution. Further clarification of chemical reaction parameters, cementation effects and multi-field coupling reaction model within the bentonite reaction system remains the focus of further researches on the chemical evolution of bentonite in the future.
-
-
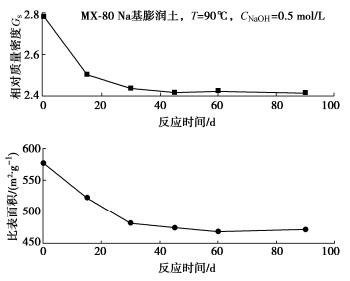
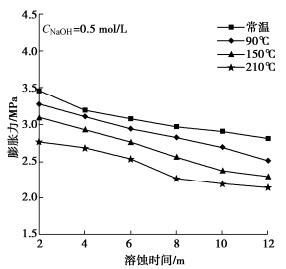
图 3 碱-热作用下膨润土基本性能变化规律[30]
Figure 3. Basic properties of bentonite under alkali-heat action
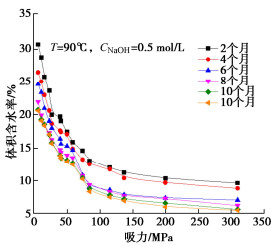
图 4 碱-热作用下膨润土持水性能随时间变化[31]
Figure 4. Water retention curves of bentonite under alkali-heat action
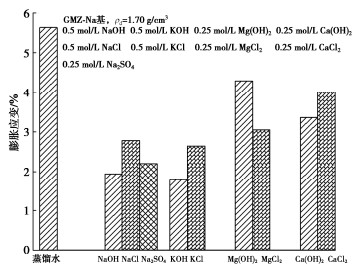
图 5 不同离子类型对膨润土膨胀变形的影响[32]
Figure 5. Effects of ion types on swelling deformation of bentonite
图 6 碱-热作用下膨润土最终膨胀力随时间变化[31]
Figure 6. Final swelling pressures of bentonite under alkali-heat action
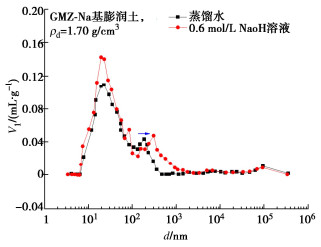
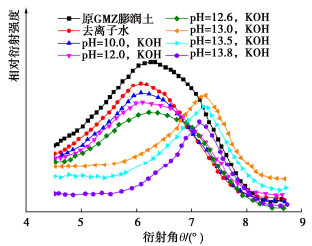
图 8 碱溶液饱和下压实膨润土孔径分布[34]
Figure 8. PSD of bentonite in high alkal isolution
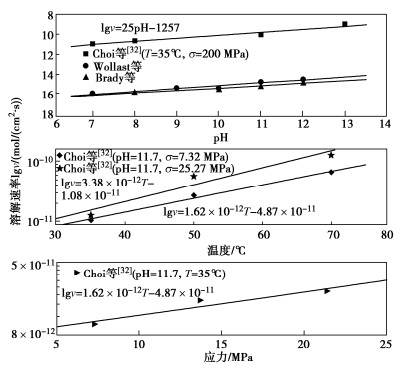
图 10 pH、温度和应力对石英矿物溶解速率的影响[37]
Figure 10. Effects of pH, temperature and stress on dissolution rate of quartz minerals
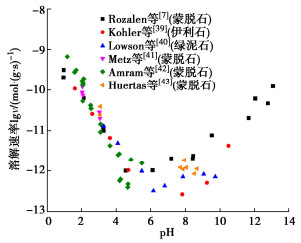
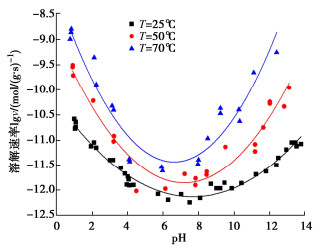
图 12 蒙脱石溶解速率随pH的变化[7]
Figure 12. Variation of dissolution rate of smectite with pH
图 13 不同碱溶液中蒙脱石d001衍射峰的变化[21]
Figure 13. Reflection peaks of smectite d001 under alkali solutions
表 1 各国处置库所选膨润土的矿物成分对比
Table 1 Mineral composition of bentonite in disposal repositories of various countries
表 2 各国处置库地下水离子成分
Table 2 Main ion components of groundwater of disposal repositories in various countries
国家 地下水类型 TDS/(g·L-1) 主要离子组成/(mmol·L-1) 文献 中国 花岗岩裂隙水 7.1 Na+(74), Ca2+(7), Cl-(53), SO42-(30) 文献[12] 西班牙 花岗岩裂隙水 0.3 Na+(0.6), Ca2+(1.0), Cl-(0.4), HCO3-(2.5) 文献[21] 法国 黏土岩孔隙水 5.7 Na+(45), Ca2+(7), Cl(40), SO42-(16) 文献[22,23] 日本 沉积岩(海水) 42.4 Na+(480), Ca2+(11), Cl-(691), SO42-(29) 文献[24] 韩国 花岗岩裂隙水 0.2 Na+(0.8), Ca2+(0.4), HCO3-(1.3), SO42-(0.1) 文献[25] 表 3 常见离子在水溶液中的直径[44]
Table 3 Diameters of common ions in aqueous solution
离子类型 Dc (Å) Ds (Å) DH (Å) H+ — (0.56) (4.56) Na+ 1.90 3.68 7.16 K+ 2.66 2.50 6.62 NH4+ 2.96 2.50 6.62 Mg2+ 1.30 6.94 8.56 Ca2+ 1.98 6.20 8.24 Fe2+ 1.50 6.88 8.56 Fe3+ 1.20 8.12 9.14 Al3+ 1.00 8.78 9.50 OH- 3.52 (0.92) (6.00) Cl- 3.62 2.42 6.64 NO3- 5.28 2.58 6.70 CO32- 5.32 5.32 7.88 SO42- 5.80 4.60 7.58 注:Dc为晶体直径;Ds为斯托克斯直径;DH为水化离子直径。 表 4 2︰1型二八面体层状黏土矿物对比[45]
Table 4 Comparison of stratified clay minerals of type 2︰1 dioctahedral
层间物质 单位晶胞携带的负电荷量X 矿物种类 无 X=0 叶腊石,滑石 阳离子或水化阳离子 0.2<X<0.6 蒙脱石,贝德石,绿脱石 0.6<X<0.9 黏粒蛭石 0.6<X<1 伊利石,水白云母
水钠云母X=1 白云母,钠云母 X=2 珍珠云母 氢氧化物 X不定 绿泥石 -
[1] 崔玉军, 陈宝. 高放核废物地质处置中工程屏障研究新进展[J]. 岩石力学与工程学报, 2006, 25(4): 842-847. doi: 10.3321/j.issn:1000-6915.2006.04.019 CUI Yujun, CHEN Bao. Recent advances in research on engineered barrier for geological disposal of high-level radioactive nuclear waste[J]. Chinese Journal of Rock Mechanics and Engineering, 2006, 25(4): 842-847. (in Chinese) doi: 10.3321/j.issn:1000-6915.2006.04.019
[2] GAUCHER E C, BLANC P. Cement/clay interactions: a review: experiments, natural analogues, and modeling[J]. Waste Management, 2006, 26(7): 776-788. doi: 10.1016/j.wasman.2006.01.027
[3] 张虎元, 李小雅, 童艳梅, 等. 高庙子膨润土在模拟水泥浸出液中的黏土矿物相变[J]. 硅酸盐学报, 2023, 51(1): 215-225. ZHANG Huyuan, LI Xiaoya, TONG Yanmei, et al. Clay phase change of gaomiaozi bentonite in simulated cement solutions[J]. Journal of the Chinese Ceramic Society, 2023, 51(1): 215-225. (in Chinese)
[4] KARNLAND O, OLSSON S, NILSSON U, et al. Experimentally determined swelling pressures and geochemical interactions of compacted Wyoming bentonite with highly alkaline solutions[J]. Physics and Chemistry of the Earth, 2007, 32(1/2/3/4/5/6/7): 275-286.
[5] BAUER A, BERGER G. Kaolinite and smectite dissolution rate in high molar KOH solutions at 35° and 80℃[J]. Applied Geochemistry, 1998, 13(7): 905-916. doi: 10.1016/S0883-2927(98)00018-3
[6] RIMSTIDT J D. Rate equations for sodium catalyzed quartz dissolution[J]. Geochimica et Cosmochimica Acta, 2015, 167: 195-204. doi: 10.1016/j.gca.2015.07.030
[7] ROZALEN M, HUERTAS F J, BRADY P V. Experimental study of the effect of pH and temperature on the kinetics of montmorillonite dissolution[J]. Geochimica et Cosmochimica Acta, 2009, 73(13): 3752-3766. doi: 10.1016/j.gca.2009.03.026
[8] RAMı́REZ S, CUEVAS J, VIGIL R, et al. Hydrothermal alteration of "La Serrata" bentonite (Almeria, Spain) by alkaline solutions[J]. Applied Clay Science, 2002, 21(5/6): 257-269.
[9] FERNÁNDEZ R, CUEVAS J, SÁNCHEZ L, et al. Reactivity of the cement–bentonite interface with alkaline solutions using transport cells[J]. Applied Geochemistry, 2006, 21(6): 977-992. doi: 10.1016/j.apgeochem.2006.02.016
[10] CHESHIRE M C, CAPORUSCIO F A, JOVÉ COLÓN C F, et al. Fe-saponite growth on low-carbon and stainless steel in hydrothermal-bentonite experiments[J]. Journal of Nuclear Materials, 2018, 511: 353-366. doi: 10.1016/j.jnucmat.2018.09.038
[11] SAVAGE D, WALKER C, ARTHUR R, et al. Alteration of bentonite by hyperalkaline fluids: a review of the role of secondary minerals[J]. Physics and Chemistry of the Earth, 2007, 32(1/2/3/4/5/6/7): 287-297.
[12] SUN Z, CHEN Y G, CUI Y J, et al. Effect of synthetic water and cement solutions on the swelling pressure of compacted Gaomiaozi(GMZ) bentonite: the Beishan site case, Gansu, China[J]. Engineering Geology, 2018, 244: 66-74. doi: 10.1016/j.enggeo.2018.08.002
[13] CUEVAS J, RUIZ A I, FERNÁNDEZ R, et al. Authigenic clay minerals from interface reactions of concrete-clay engineered barriers: a new perspective on Mg-clays Formation in alkaline environments[J]. Minerals, 2018, 8(9): 362. doi: 10.3390/min8090362
[14] PEKALA M, WERSIN P, PASTINA B, et al. Potential impact of cementitious leachates on the buffer porewater chemistry in the Finnish repository for spent nuclear fuel-A reactive transport modelling assessment[J]. Applied Geochemistry, 2021, 131: 105045. doi: 10.1016/j.apgeochem.2021.105045
[15] MITCHELL J K, SOGA K. Fundamentals of Soil Behavior[M]. New York: Wiley, 2005.
[16] GARCÍA-ROMERO E, LORENZO A, GARCÍA-VICENTE A, et al. On the structural formula of smectites: a review and new data on the influence of exchangeable cations[J]. Journal of Applied Crystallography, 2021, 54(Pt 1): 251-262.
[17] CHEN Y G, DONG X X, ZHANG X D, et al. Combined thermal and saline effects on the swelling pressure of densely compacted GMZ bentonite[J]. Applied Clay Science, 2018, 166: 318-326. doi: 10.1016/j.clay.2018.10.001
[18] SUN D A, ZHANG L, LI J, et al. Evaluation and prediction of the swelling pressures of GMZ bentonites saturated with saline solution[J]. Applied Clay Science, 2015, 105/106: 207-216. doi: 10.1016/j.clay.2014.12.032
[19] KARNLAND O, OLSSON S, NILSSON U. Mineralogy and Sealing Properties of Various Bentonites and Smectite-Rich Clay Materials[R]. Stockholm: Svensk Kärnbränslehantering Ab, 2006.
[20] LEE J O, LIM J G, KANG I M, et al. Swelling pressures of compacted Ca-bentonite[J]. Engineering Geology, 2012, 129/130: 20-26. doi: 10.1016/j.enggeo.2012.01.005
[21] VILLAR M V, IGLESIAS R J, GUTIÉRREZ-ÁLVAREZ C, et al. Hydraulic and mechanical properties of compacted bentonite after 18 years in barrier conditions[J]. Applied Clay Science, 2018, 160: 49-57. doi: 10.1016/j.clay.2017.12.045
[22] WANG Q. Hydro-Mechanical Behaviour of Bentonite- Basedmaterials Used for High-Level Radioactive Waste Disposal[D]. Paris: Ecole Des Ponts Paris Tech, 2012.
[23] 马婧, 陈永贵, 刘聪, 等. 化学作用下压实膨润土膨胀力响应机制研究进展[J]. 岩土工程学报: 1-10. MA Jing, CHEN Yong-gui, LIU Cong, 等. Research progress on the swelling pressures mechanisms of compacted bentonite under chemical conditions[J]. Chinese Journal of Geotechnical Engineering: 1-10. (in Chinese)
[24] KOMINE H, YASUHARA K, MURAKAMI S. Swelling characteristics of bentonites in artificial seawater[J]. Canadian Geotechnical Journal, 2009, 46(2): 177-189. doi: 10.1139/T08-120
[25] KIM S S, BAIK M H, KANG K C. Solubility of neptunium oxide in the KURT (KAERI Underground Research Tunnel) groundwater[J]. Journal of Radioanalytical and Nuclear Chemistry, 2009, 280(3): 577-583. doi: 10.1007/s10967-009-7481-y
[26] 童艳梅, 张虎元, 周光平, 等. 高庙子膨润土中蒙脱石碱性溶蚀的矿物学证据[J]. 岩土力学, 2022, 43(11): 2973-2982. TONG Yanmei, ZHANG Huyuan, ZHOU Guangping, et al. Mineralogical evidence of alkaline corrosion of montmorillonite in GMZ bentonite[J]. Rock and Soil Mechanics, 2022, 43(11): 2973-2982. (in Chinese)
[27] 陈宝, 张会新, 陈萍. 高碱溶液对高庙子膨润土侵蚀作用的研究[J]. 岩土工程学报, 2013, 35(1): 181-186. http://cge.nhri.cn/article/id/14929 CHEN Bao, ZHANG Huixin, CHEN Ping. Erosion effect of hyper-alkaline solution on Gaomiaozi bentonite[J]. Chinese Journal of Geotechnical Engineering, 2013, 35(1): 181-186. (in Chinese) http://cge.nhri.cn/article/id/14929
[28] KAUFHOLD S, DOHRMANN R, SANDÉN T, et al. Mineralogical investigations of the first package of the alternative buffer material test–I. Alteration of bentonites[J]. Clay Minerals, 2013, 48(2): 199-213. doi: 10.1180/claymin.2013.048.2.04
[29] KAUFHOLD S, DOHRMANN R, WALLIS I, et al. Chemical and mineralogical reactions of bentonites in geotechnical barriers at elevated temperatures: review of experimental evidence and modelling progress[J]. Clay Minerals, 2023, 58(3): 280-300. doi: 10.1180/clm.2023.26
[30] 曾召田, 张瀚彬, 吕海波, 等. 高温强碱条件下膨润土物理性能的时效性[J]. 土木与环境工程学报(中英文): 1-8. ZENG Zhaotian, ZHANG Hanbin, LÜ Haibo, et al. Aging effect on physical properties of bentonite under high temperature-strong alkaline conditions[J]. Journal of Civil and Environmental Engineering: 1-8. (in Chinese)
[31] 郭招群. 碱—热耦合作用下膨润土水力演化特征研究[D]. 绵阳: 西南科技大学, 2018. GUO (QIAO Zhaoqun. Study on Hydraulic Evolution Characteristics of Bentonite under Alkali-Heat Coupling[D]. Mianyang: Southwest University of Science and Technology, 2018. (in Chinese)
[32] 陈龙. 复杂化学环境下膨润土膨胀变形研究[D]. 绵阳: 西南科技大学, 2021. CHEN Long. Study on Swelling Deformation of Bentonite in Complex Chemical Environment[D]. Mianyang: Southwest University of Science and Technology, 2021. (in Chinese)
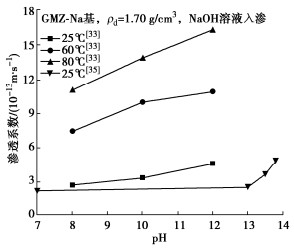
[33] YE W M, ZHENG Z J, CHEN B, et al. Effects of pH and temperature on the swelling pressure and hydraulic conductivity of compacted GMZ01 bentonite[J]. Applied Clay Science, 2014, 101: 192-198. doi: 10.1016/j.clay.2014.08.002
[34] 陈宝, 张会新, 陈萍. 高碱溶液入渗对GMZ膨润土微观孔隙结构的影响[J]. 浙江大学学报(工学版), 2013, 47(4): 602-608. CHEN Bao, ZHANG Huixin, CHEN Ping. Influence of hyper-alkaline solution infiltration on microscopic pore structure of compacted GMZ bentonite[J]. Journal of Zhejiang University (Engineering Science), 2013, 47(4): 602-608. (in Chinese)
[35] CHEN B, GUO J X, ZHANG H X. Alteration of compacted GMZ bentonite by infiltration of alkaline solution[J]. Clay Minerals, 2016, 51(2): 237-247. doi: 10.1180/claymin.2016.051.2.10
[36] JORDI Cama, JIWCHAR Ganor. Natural and Engineered Clay Barriers[M]. Amsterdam: Elsevier, 2015: 101-153.
[37] CHOI J H, KIMOTO K, ICHIKAWA Y. Quartz dissolution experiments at various pH, temperature and stress conditions: CLSM and ICP-AES investigations[J]. Environmental Earth Sciences, 2012, 66(8): 2431-2440. doi: 10.1007/s12665-011-1467-0
[38] UETA S, SATOH H, KATO H, et al. Interlayer dissolution of montmorillonite observed by internal refraction interferometry[J]. Journal of Nuclear Science and Technology, 2016, 53(2): 184-191. doi: 10.1080/00223131.2015.1029556
[39] KÖHLER S J, DUFAUD F, OELKERS E H. An experimental study of illite dissolution kinetics as a function of ph from 1.4 to 12.4 and temperature from 5 to 50℃[J]. Geochimica et Cosmochimica Acta, 2003, 67(19): 3583-3594. doi: 10.1016/S0016-7037(03)00163-7
[40] LOWSON R T, BROWN P L, COMARMOND M C J, et al. The kinetics of chlorite dissolution[J]. Geochimica et Cosmochimica Acta, 2007, 71(6): 1431-1447. doi: 10.1016/j.gca.2006.12.008
[41] METZ V, AMRAM K, GANOR J. Stoichiometry of smectite dissolution reaction[J]. Geochimica et Cosmochimica Acta, 2005, 69(7): 1755-1772. doi: 10.1016/j.gca.2004.09.027
[42] AMRAM K, GANOR J. The combined effect of pH and temperature on smectite dissolution rate under acidic conditions[J]. Geochimica et Cosmochimica Acta, 2005, 69(10): 2535-2546. doi: 10.1016/j.gca.2004.10.001
[43] HUERTAS F J, CABALLERO E, JIMÉNEZ DE CISNEROS C, et al. Kinetics of montmorillonite dissolution in granitic solutions[J]. Applied Geochemistry, 2001, 16(4): 397-407. doi: 10.1016/S0883-2927(00)00049-4
[44] NIGHTINGALE E R Jr. Phenomenological theory of ion solvation. effective radii of hydrated ions[J]. The Journal of Physical Chemistry, 1959, 63(9): 1381-1387. doi: 10.1021/j150579a011
[45] 方邺森. 黏土矿物的分类[J]. 海洋地质与第四纪地质, 1985(2): 125-127. FANG Yesen. Classification of clay minerals[J]. Marine Geology & Quaternary Geology, 1985(2): 125-127. (in Chinese)
[46] FERNÁNDEZ R, CUEVAS J, MÄDER U K. Modeling experimental results of diffusion of alkaline solutions through a compacted bentonite barrier[J]. Cement and Concrete Research, 2010, 40(8): 1255-1264. doi: 10.1016/j.cemconres.2009.09.011
[47] BAUER A, VELDE B. Smectite transformation in high molar KOH solutions[J]. Clay Minerals, 1999, 34(2): 259-273. doi: 10.1180/000985599546226
[48] VAN DE KAMP P C. Smectite-illite-muscovite transformations, quartz dissolution, and Silica release in shales[J]. Clays and Clay Minerals, 2008, 56(1): 66-81. doi: 10.1346/CCMN.2008.0560106
[49] 张明, 谢敬礼. 高放处置罐铁释放诱发膨润土矿物相变研究进展[J]. 岩石矿物学杂志, 2021, 40(4): 778-785. doi: 10.3969/j.issn.1000-6524.2021.04.009 ZHANG Ming, XIE Jingli. A review on the study of mineral phase transformation of bentonite induced by iron release in the high-level radioactive waste repository[J]. Acta Petrologica et Mineralogica, 2021, 40(4): 778-785. (in Chinese) doi: 10.3969/j.issn.1000-6524.2021.04.009
[50] OSACKý M, ŠUCHA V, CZíMEROVá A, et al. Reaction of smectites with iron in aerobic conditions at 75℃[J]. Applied Clay Science, 2013, 72: 26-36. doi: 10.1016/j.clay.2012.12.010
[51] WYPYCH F, FREITAS R A D. Developments in Clay Science[M]. Amsterdam: Elsevier, 2022: 3-35.
[52] HE X, PAN Y, CASERES L, et al. Assessment of Aging Mechanisms for Concrete Exposed to Outdoor Air and Groundwater or Soil in Spent Nuclear Fuel Dry Storage Systems[R]. Houston: Nace International, 2018.
-
其他相关附件



 下载:
下载: